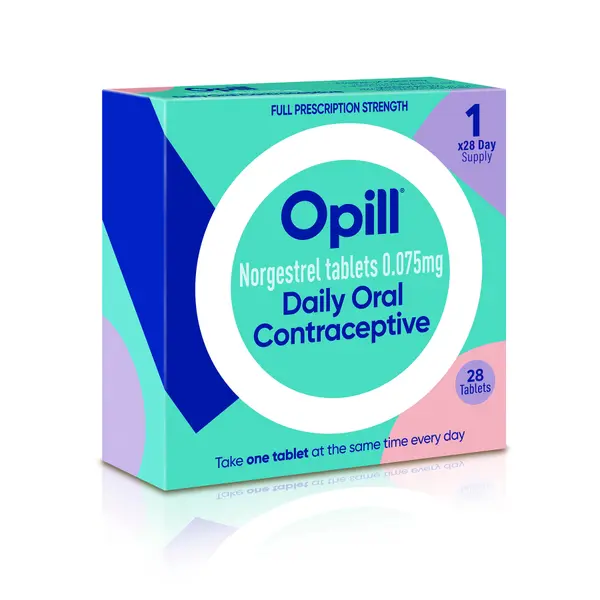
In a groundbreaking decision, the Food and Drug Administration (FDA) has granted approval for Opill, the first daily oral contraceptive pill to be available without a prescription. This landmark event comes at a time when certain U.S. states have been attempting to restrict access to birth control and abortion.
Opill, containing norgestrel and available in tablet form, will now be sold over the counter in various venues, including online markets, drug stores, convenience stores, and grocery stores. The FDA’s Center for Drug Evaluation and Research director, Patrizia Cavazzoni, stated that this approval provides millions of people in the United States with a nonprescription daily oral contraceptive option, which is both safe and expected to be more effective than current nonprescription methods in preventing unintended pregnancies.
Manufactured by Perrigo, with headquarters in Dublin, Opill is categorized as a “progestin-only” pill, utilizing a synthetic version of the hormone progesterone to prevent pregnancy. This approval has been hailed as a momentous day for women’s health nationwide, as it has the potential to significantly enhance access to contraception.
Perrigo President and CEO Patrick Lockwood-Taylor expressed excitement about the transformative impact Opill could have on women’s access to contraception. The exact timeline for the availability of Opill, sold in boxes containing a 28-day supply, will be determined by Perrigo. The company anticipates making the product available in stores and online during the first quarter of 2024. Pricing details are yet to be released.
It is important to note that while Opill becomes accessible without a prescription, other approved formulations and dosages of oral contraceptives will still require a prescription as per the FDA’s statement.
A global media for the latest news, entertainment, music fashion, and more.